Chemistry
Chemistry programs for Years 11-12, mapped to the Australian National Curriculum and tailored to your needs.

Our Chemistry Tutoring provides Senior students with the expert tuition they need to succeed in this challenging subject
Expert tutors
That are friendly and relatable.
Quality content
Created by teaching specialists.
Delivered online
From the comfort of home at a time that suits you.
Find a specific Chemistry program
We offer personalised tutoring programs mapped to the Australian National Curriculum and aligned with the modules you’re covering in class.
Currently in years 9 or 10?Get a head start on Senior Chemistry by checking out our Year 11 programs.
See How Cluey Learning Works!
How our online face-to-face tutoring works

Complete Questionnaire
Fill out our 2-minute questionnaire to provide information about your child's goals, and we'll present some program options and pricing.

Have a chat
We'll reach out to you to discuss your child's needs in detail and set up their first tuition session with one of our qualified and friendly tutors.

Meet, greet and evaluate
Your child attends their first session, where we'll evaluate their current learning and identify which of our highly-effective curriculum programs, designed by our expert educators, best fits their goals and needs.

It's go-time
Your child begins their Cluey Learning journey, working through their program with the support and guidance of our experienced and dedicated tutors.

Smooth sailing
You monitor and manage your child's enrolment through your Cluey Hub, where we provide written feedback, progress reporting and session recordings. You can also adjust your schedule, change tutors and purchase session bundles.
20% Off a trial session - limited spots available
Find a specific Chemistry program Trial Session
Looking for specific help?
We’re all made of atoms
Chemistry might not be something you think of every day, but every part of the world you touch, see, and experience has a chemical component and interaction your student needs to know about.
At Cluey, our skilled Chemistry tutors all have advanced knowledge of the topic, giving your student personalised access to answers, explanations and challenges that can help them succeed at school.
How does Cluey Chemistry tutoring work?
Cluey tutors teach face-to-face in a live online session that includes audio, video and the use of our collaborative whiteboards.
Customised to suit your student’s needs, we work through any problem areas they’re struggling with (or provide topic reinforcement or extension programs where required).
- Discussion (about the problem)
- Demonstration of how to solve it
- Challenge for the student to solve, with help if needed.
We’ll even provide practice questions to work on between sessions, so they can master the topics they need to learn.
We've got all your Chemistry learning goals covered
Address a specific individual need
Perhaps the class room just isn't meeting all their learning needs or you want to prepare for a specific assessment.
Let's build a learning program for
Tutoring programs for the optimum level of concentration

Meet Dr Selina Samuels
Dr Samuels and her Education team developed Cluey's unique CENTRE approach, and have created interactive learning programs that map to the curriculum and allow students to work at their own level and pace.
Chemistry Tutoring Program Based on the National Curriculum
Our Chemistry program has everything you require to master the subject. We work through the syllabus with you, starting with what you’re currently learning at school, and adjust the program based on your specific needs and pace of learning.
Meet some of our 2,521+ expert Chemistry tutors
Our tutors are all qualified teachers, exceptional ATAR achievers or experts in their respective fields.

Brayn
NSW University Student (Bachelor of Medical Science)
Chemistry: Senior
English: Secondary & Senior
Bryan graduated in 2018 with an ATAR of 94.45 and is currently studying a Bachelor of Medical Science. With prior experience as a tutor Bryan is passionate about developing a student's critical analysis, written and oral communication skills as they work towards acquiring educational independence. Bryan believes in fostering a positive and encouraging learning environment where students engage in collaborative discussions and feels that all students can reach their potential

Nikhil
QLD University Student (Bachelor of Medical Studies / Doctor of Medicine)
Mathematics: Secondary
Chemistry: Senior
Nikhil holds a Master of Teaching (Primary) from the University of Western Sydney. He currently works as a Casual Teacher at various schools. As an educator, he aims to instil the value of education in his students and offer them rich and varied learning experiences that will enhance their performance as well as remain memorable to them.

Nighean
Mathematics: Secondary
Chemistry: Senior
Nighean completed her Bachelor of Science at Monash University in 2018 and has experience with special needs children. Nighean believes in fostering a positive and encouraging learning environment and feels with the right support, students can reach their potential.
How does our approach to learning work?
Tailored tutoring with a plan
The learning program
Structured around your child's individual needs and goals, our learning programs adapt over time to build confidence and offer the right help at the right time.
Quality content
- Developed by our experienced education team, the content in our learning programs is structured according to your child's skill level and individual needs.
- We use a range of approaches to meet the needs of different students. Our approach is always clear and unambiguous, and designed to help your child thrive.

Tutoring sessions
- Live, face-to-face and online
- Expert tutors matched to your child's needs
- Demonstration, guided exercises and reviews
- Designed to be fun and engaging
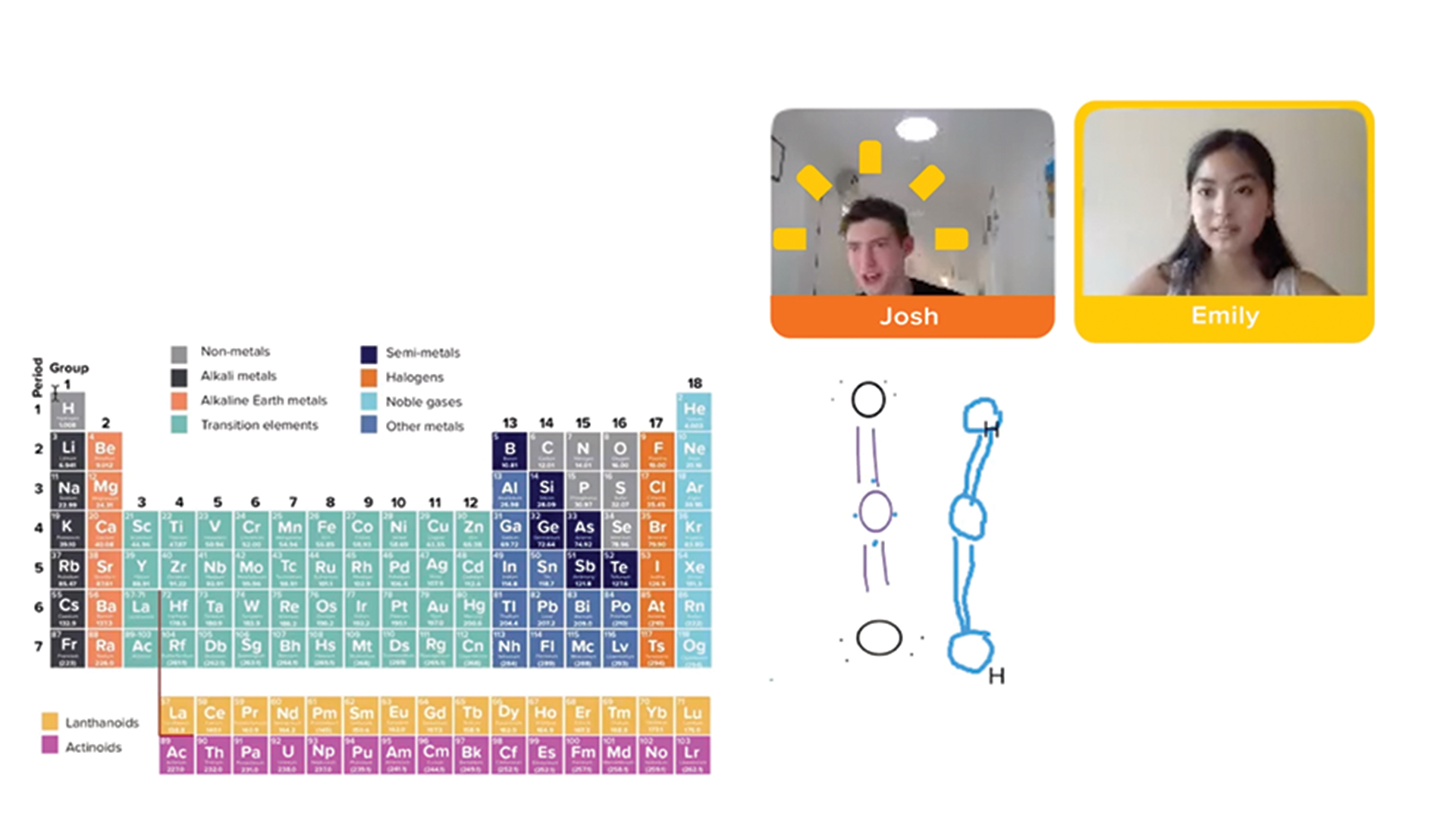
View a 1-to-1 Chemistry Session

Practice exercises
Optional practice questions and exercises to work through between sessions.

Regular reporting
Feedback after every session helps you keep track of your child's progress.


Learn from home (or anywhere) via our online platform.

Schedule sessions when they suit you, between 7am and 10pm, 7 days per week.

Reschedule easily when things get busy
From step one to step done, we're with your child for their entire learning journey.

Session recordings allow your child to revise at any time.

Progress reports ensure your child is getting the right help at the right pace.

Guided tutoring helps your child realise their potential.
Let's build a learning program for
We've helped thousands of Aussie families
From catching up, building confidence and excelling in school, parents and students alike agree that Cluey works.

82% of Cluey parents agree their child’s grades have improved

85% of Cluey parents agree their child is more confident
Cluey is helping students all over Australia
Read reviewsIncreased confidence and grade average
“My son Ben has had 13 on line chem sessions with Cluey leading up to his final exams. I chose Cluey over a range of online and personal tutoring options because they talked to Ben's needs and offered high calibre tutors with a depth of knowledge of current curricula. The on line platform is also excellent. Ben's grade average has progressively increased and he is looking forward to his final exam with the confidence he was lacking months ago.”
As featured in
Our report card is in and feedback is positive.
(Click a logo to read more)
Our Partners
We partner with Australian charities, education and community organisations to assist students with their learning and support educational research.
Learn more about the Cluey Impact program

Get your personalised program, pronto!
Enter a few details below
School Year
Please select a year level
Cluey 1 Hour Tutoring Sessions
Weekly learning through live tutoring sessions, feedback & practice
Each student’s individual learning journey is supported through a series of live face-to-face sessions with an expert tutor matched to the student's needs. We cover all the theory and examples needed to ensure comprehension, and our sessions are designed to be engaging and encouraging.
Our expert tutors offer guidance through demonstration and worked examples and assign targeted practice questions to help students master the topics and concepts covered.
After each session, personalised feedback is provided to help students and parents track their progress.
Session breakdown
First 5 Minutes
Establishing the session theme, why are we here?
- Discuss student’s areas of focus (if first session)
- Review previous session's assigned practice (if subsequent session)
- Set session learning goals
45 Minutes
During the session
- Work through exercises based on the topics and concepts for each learning goal
- Tutor demonstrates, guides and explains concepts
- Work through any challenges
- Students are encouraged to explain their thinking to clarify their comprehension
Closing 5 Minutes
Reflection and looking ahead
- Reflect on what has been achieved in the session
- Set practice questions
Post-session 5 minutes
Tutor written feedback
- Tutor provides personalised written feedback about the session to help track progress